Xcheck Quality Assurance
Team Cross Check QA is a dedicated group of professionals specializing in quality assurance, product certification, and firm registration with government and regulatory agencies. We bring deep expertise and a commitment to precision, helping businesses navigate complex compliance landscapes with confidence.

Laboratory search made simple
Laboratory testing plays an essential role throughout the entire product life cycle in research and development, qualification, manufacturing and operations. Analytical testing ensures the safety and efficacy of new materials, components and systems, performance and endurance of new products during design, quality control during manufacturing or construction, to analyse failure, identify causes and prevent new occurrences. Always verify the laboratory’s NABL accreditation status and scope.
XcheckQA has developed an advanced Lab Finder Application which empowers users to find a perfect NABL-accredited laboratory for their need using the following filters.
Type of Laboratories
Laboratory Name
Material-specific testing
Specification/Test Procedure
NABL Accreditation Certificate
Location
Parameter/Characteristic
Instrument type & measurement range
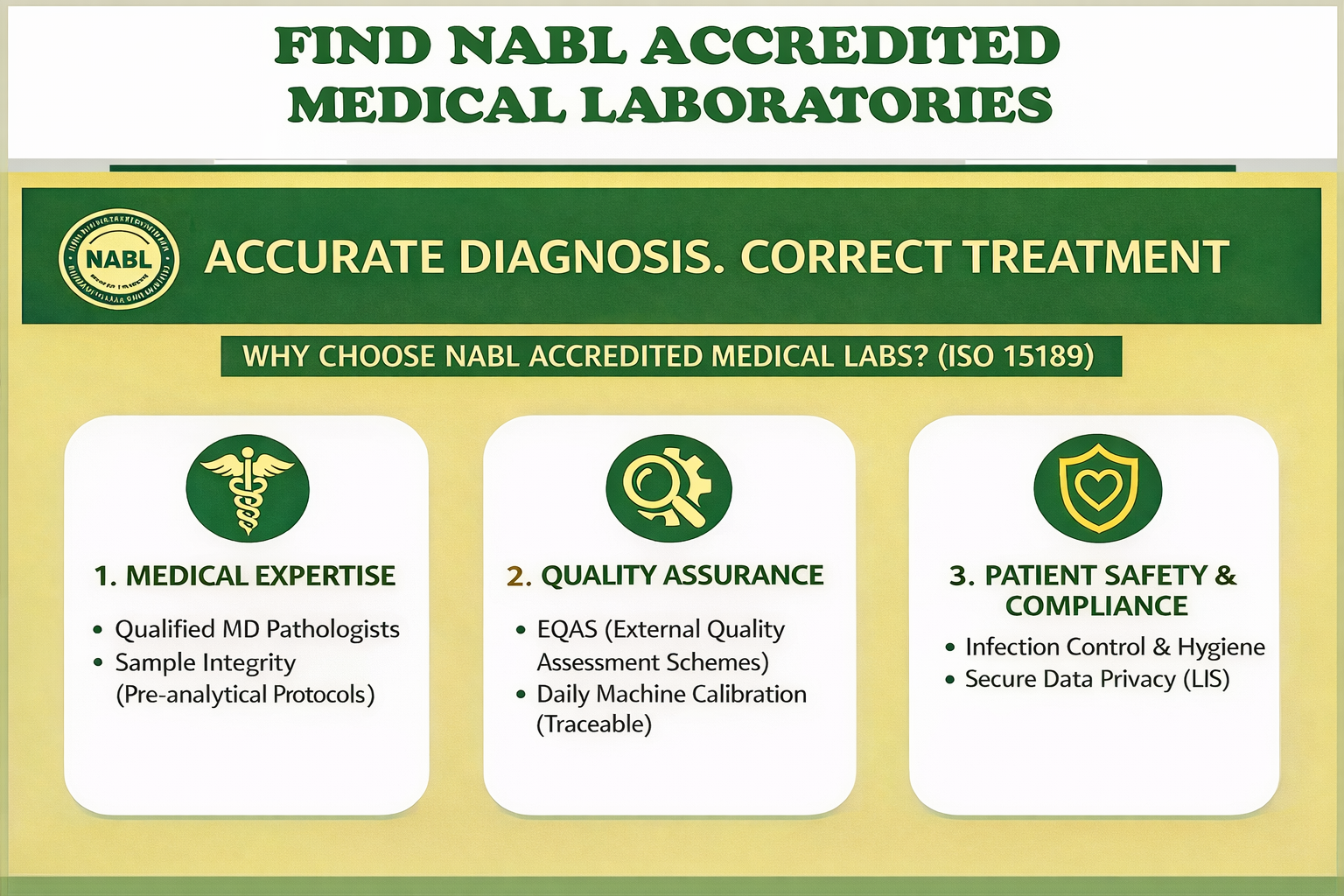
Medical Laboratories
Medical laboratories are integral components of the healthcare system, playing a pivotal role in disease diagnosis, monitoring, and treatment. NABL accredited facilities are equipped with state-of-the-art instrumentation and employ highly skilled laboratory professionals to analyse patient samples and generate critical diagnostic information leading to increased accuracy, efficiency, and the ability to detect a wide range of diseases. Laboratory tests are essential for detecting biomarkers, pathogens, genetic mutations, and other indicators of disease. Selection a correct NABL accredited medical laboratory enable precise and personalized diagnostic approaches.
Testing Laboratories
Analytical testing and quality assurance testing play a critical role, ensuring that products meet industry benchmarks and regulatory requirements. Compliance to the standards is a key driver of success and sustainability for businesses. Analytical testing ensures that products meet regulatory standards by thoroughly examining their composition, properties, and performance. Selecting a NABL accredited laboratory ensure compliance with strict rules, regulations.


Calibration Laboratories
In industries where precision, reliability, and accuracy are critical, laboratories play an essential role. Measurement is the key in any of the field whether it’s for medical diagnostics, product quality control, environmental monitoring, the credibility of a measurement results directly impacts decision-making. Calibration is compulsory for measuring instruments to ensure the accuracy and reliability of measurements. Always verify the laboratory’s NABL accreditation status and scope as per the type, range and measurement capability of the instrument to ensure accuracy and reliability.
Proficiency Testing Laboratories
Proficiency Testing (PT) programs involve the distribution of samples with known parameters to participating laboratories. These laboratories analyse the samples and report their results, which are then compared with results from other laboratories to assess performance and identify areas for improvement. Many industries require laboratories to participate in PT programs to evaluate testing accuracy and method competence for specific analytes, ensuring compliance with regulatory requirements and maintaining accreditation. Through regular participation in PT programs, laboratories can demonstrate the accuracy, reliability, and validity of their results. It is essential to always verify the NABL accreditation status and the scope of its Proficiency Testing programs.


Reference Material Producers
A Reference Material Producer is an organization that develops, characterizes, and supplies reference materials with known and well-established property values. These materials are used by laboratories for method validation, calibration, quality control, and proficiency testing to ensure the accuracy and traceability of measurement results. Competent RMPs operate in accordance with ISO 17034 requirements and provide reference materials that are homogeneous, stable, and accompanied by proper documentation, including certificates and uncertainty statements. It is essential to always verify the NABL accreditation status and the scope of Reference Material Producers (RMP).
Central Government Health Scheme (CGHS)
CGHS is providing comprehensive medical care to the Central Government employees and pensioners. All the beneficiaries intending to avail medical facilities are advised to ensure before availing the facility that the concerned hospital/centre is under empanelment of CGHS on that date to avoid rejection/deduction of reimbursement.


CGHS Empanelled Hospital
The comprehensive list categorizes hospitals into General Hospitals, Eye Hospitals, Dental Hospitals, and Diagnostic Centres to provide a wide range of healthcare options for beneficiaries. Individuals covered under the CGHS have access to a diverse network of healthcare providers for their medical needs, promoting quality care and convenience for all.
CGHS Empanelled Diagnostic Centres
A CGHS empanelled medical lab is a diagnostic centre that is officially approved and listed by the CGHS authority to provide subsidized or cashless diagnostic services to CGHS cardholders. These labs meet high-quality standards in testing and diagnostics and are a lifeline for thousands of government employees and pensioners.

BIS Approved Laboratories
BIS operates various conformity assessment schemes. Under these schemes, BIS grants licenses/registrations to such manufacturers who are capable of producing goods conforming to relevant Indian Standards, on continuous basis. To support these schemes, which requires testing of products on regular basis for checking conformity to the relevant Indian Standards, BIS has established a network of eight laboratories. Find BIS approved Testing facility and Testing charges
Quality Assurance
At XcheckQA, our team reviews regulatory frameworks, collaborates across departments to gather meaningful data, and conducts in-depth evaluations of existing quality control systems. This helps us pinpoint strengths and address vulnerabilities, creating space for smarter, more effective operational improvements.

Regulatory Advisory
At Xcheck, we guide companies through complex manufacturing protocols and mandatory regulations. Our team validates operational systems, identifies defects, prepares essential documentation for product validation, and recommends corrective actions while upholding regulatory standards and aligning with your organization’s mission and policies.
Documentation
Documents Vetting is the critical step of reviewing and validating technical documents, specifications, and contracts to ensure accuracy, compliance, and readiness for inspection, Registration and approvals. XcheckQA assists manufacturers, exporters, and suppliers in creating and refining essential technical documents for quality assurance and regulatory compliance. Our expertise helps minimize inspection delays and ensure accurate, verifiable records across operations.


Registration of Manufacturer with Government & PSUs
Manufacturers supplying products to Defence (Army, Navy, Air Force, DPSUs, OFB, etc.), Railways, PSU and other government agencies in India must undergo a formal registration with
MSME (Micro, Small, Medium enterprises)
NSIC (National Small Industries Corporation)
Defence PSUs (BEL, HAL, BEML, BDL, etc.)
DRDO (Defence Research & Development Organisation)
DGQA (Directorate General of Quality Assurance),
DGAQA (Directorate General of Aeronautical Quality Assurance),
RDSO (Research Designs & Standards Organisation)
Lab Finder App
Our innovative Lab Finder App closes the gap between test requirements and accredited lab capabilities, saving users time, cost, and frustration.
XcheckQA Application is a specialized lab-finder platform developed to streamline the search for NABL accredited medical, industrial, and calibration laboratories across all sectors.
Application facilitates seamless interaction between different stakeholders—such as industrial clients, certified laboratories, and consultants.
User can send structured queries directly to registered labs, share sample details, testing requirements, and receive responses, quotations, timelines and confirmations—all within the app
Download XcheckQA Lab Finder App available on both Android and iOS platforms

Services to Laboratories
Crosscheck QA connect testing and calibration service users to NABL accredited testing/calibration service providers.
Members laboratories will receive unlimited queries directly from the customers as per NABL scope.
Laboratories can send estimate and sample requirement to the customers through Xcheck online portal.
Laboratories can also generate internal documents and internal reports through XcheckQA online portal.
Final reports also be generated online or uploaded through online portal direct to the customers avoiding delay.
XcheckQA ensures faster communication, improved transparency and end to end digital management of laboratories services.
Start your Free trial
Grow stronger together & manage data. Everything in one place
Our customers love us
Check out what they say about us

Mission
At XCheckQA, our mission is to deliver excellence in performance through rigorous quality assurance, structured audits, data-driven process validation and ensuring precision, reduced defects, safety, compliance, and consistent product excellence enabling sustainable production growth.
Vision
To be a trusted quality partner and recognized leading catalyst in quality assurance and testing services, renowned for our unwavering commitment to hand hold our clients to achieve flawless products and seamless user experiences. We aspire to set the benchmark for precision and excellence.








